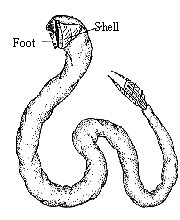
|
CLASS
BIVALVIA (PELECYPODA)
|
(Bi-valv
i-a) (pely-cy-poda)
All of
the Taxonomy is
UNDER CONSTRUCTION - Plus
Images are being added and the article is being updated Nov. 24, 2003
Latin;
bi=two - two plates (Two halves to the shell)
Pele=hatchet pod=foot hatchet foot (shape)
 |
The
Pelecypoda, Bivalva or Lamellibranchia (Latin for leaf-gill) (the only
class with three names!!) is comprised of molluscs known more commonly
as just �bivalves �, because they have two separate halves
to their shells. They all have two-part shells, hinged dorsally. The
head is greatly reduced in size and their foot is laterally compressed.
Their mantle cavity is the largest of all known molluscs. Their
gills tend to be very large and not only function for respiration, but
aid in food-collecting as well.
|
| |
|
|
Most bivalves have evolved to become burrowers. They have left the hard
substratum of their ancestors and have learned to inhabit the massive
mud, silt and sand bottoms of our oceans and freshwaters. Some bivalves
do however live on, or most often in hard substrata such as clay, rocks
and wood. These have become sessile (i.e., once adult, they don't
move), or borers (example - the famous �shipworms �- of various families,
including Litihophagidae (litho= wood, phag = eat: wood eater).
|
Teredo navalis
(Linnaeus, 1758)
Shipworms |
|
NOTE:
Shipworms are not a worm at all, but a greatly elongated clam . Its two
shells, enclosing only the front end of the body, function as a tool,
rather than a protective covering; their ridged and roughened surfaces
are used for boring. They are actually a boring clam. Christened by mariners,
"termites of the sea," shipworms are parasitic mollusks that
thrive in and upon submerged wooden structures, including pilings, bulkheads
and the untreated hulls of boats. They are quite destructive and have
actually sunk many a wooden ship of old. As they tunne and eat the wood,
their tunnel diameter actually increase in diameter due to their growth.
|
Most bivalves are marine, and of these the majority live in the littoral, or
intertidal zone. However, some species are found in the deepest abyssal zones
of the oceans. Some bivalve species and groups have adapted to living
in brackish and freshwater environments. These are found in the freshwater families
of the Unionidae (These will be discussed further down the page). Also, some
of the "true" mussels (family Mytilidae) such as the infamous
Zebra mussels are also found
in brackish and fresh water. Some of the bivalves lead a commensural
life style: living with other marine inhabitants, while still others
have evolved to become parasites.
During periods of low tide or drought, exposed fresh-water bivalves retain precious
moisture by keeping totally inactive (which is called "aestivation": their metabolic rate drops
to zero, so they can last a long time without water!), retaining fluid within
their mantle cavity.
Bivalves have long played a role in feeding the world's population. Another
area where they are important is for man's ornamentation and adornment
throughout the ages. Pearls are very economically important as a jewelry
item, and many bivalve shells are used in various decorative ways. (See the
Man and Mollusc article for details
on the many interesting uses man has put molluscs, including bivalves to, over
the centuries).
Bivalves are highly specialized not only in their shape, but in their physiology
as well. Because of this specialization, most remain living in and
on "soft bottoms" such as sand, silt and well-oxygenated mud.
|
Taxonomy
of Bivalves
|
|
There
are a few good taxonomic data bases for one to choose from on the
world wide web. Even scientists cannot agree one hundred percent
on who is right. With the advent of DNA testing and other scientific
equipment that is now available to use to delve into molluscs ever
deeper; changes will be continually made as to what order, family,
etc. that a mollusc belongs to.
I
will show just two of the data bases that I used to help me rewrite
my section on bivalves. I will use the OBIS data base to further
discuss the deferent subclasses in this article.
|
OBIS:
This
database was compiled by teams at the Academy of Natural Sciences,
the Australian Museum, the Muséum National d'Histoire Naturelle,
and the California Academy of Sciences, with support from the
Alfred P. Sloan Foundation, the National Oceanographic Partnership
Program, and the Australian Biological Resources Study. This database
is part of the Ocean Biogeographic Information System.
|
World
Biodiversity Database:
ETI,
the Expert Center for Taxonomic Identification, is a not for profit
foundation, dedicated to improve on a global scale the quantity,
quality and accessibility of taxonomic information, based on an
initiative of UNESCO.
|
- Subclass: Protobranchia
- Nuculoida
- Solemyoida
- Solemyidae
Gray, 1889
- Nucinellidae
Vokes, 1956
|
- Subclass Protobranchia
- Order Nuculoida
- Family
Nuculanidae
- Family
Nuculidae
- Family
Phaseolidae
- Family
Yoldiidae
|
- Subclass: Pteriomorphia
- Mytiloida
- Mytilidae
Rafinesque, 1815
- Arcoida
- Pterioida
- Limoida
- Ostreoida
|
- Subclass Pteromorphia
- Order Arcoida
- Family Arcidae
- Family Glycymerididae
- Family Noetidae
- Family Arcidae
- Family Glycymerididae
- Family Noetidae
- Order Mytiloida
- Family Mytilidae
- Family Pinnidae
- Order Ostreoida
- Order Pterioida
- Family Anomiidae
- Family Limidae
- Family Pectinida
|
- Subclass: Palaeoheterodonta
|
- Subclass Palaeoheterodonta
¬
- Order Unionoida
¬
- Superfamily
Unionoidea
- Family
Margaritiferidae
- Family
Unionidae
|
- Heterodonta
- Veneroida
- Chamoidea
- Lucinoidea
- Galeommatoidea
- Cyamioidea
- Carditoidea
- Crassatelloidea
- Cardioidea
- Tridacnoidea
- Mactroidea
- Solenoidea
- Tellinoidea
- Arcticoidea
- Glossoidea
- Corbiculoidea
- Veneroidea
- Myoida
- Myoidea
- Gastrochaenoidea
- Hiatelloidea
- Pholadoidea
|
- Subclass Heterodonta
- Order Veneroida
- Family Arcticidae
- Family Astartidae
- Family Cardiidae
- Family Donacidae
- Family Kelliidae
- Family Lasaeidae
- Family Leptonidae
- Family Lucinidae
- Family Mactridae
- Family Montacutidae
- Family Petricolidae
- Family Pharidae
- Family Psammobiidae
- Family Scrobiculariidae
- Family Semelidae
- Family Solecurtidae
- Family Solenidae
- Family Tellinidae
- Family Thyasiridae
- Family Turtoniidae
- Family Ungulinidae
- Family Veneridae
- Order Myoida
- Family Corbulidae
- Family Hiatellidae
- Family Myidae
- Family Pholadidae
- Family Teredinidae
- Family Xylophagidae
- Superfamily
Dreissenoidea
- Superfamily
Sphaerioidea
- Family Pisidiidae
- Family Sphaeriidae
|
- Subclass: Anomalodesmata
- Pholadomyoida
- Pholadomyoidea
- Thracioidea
- Clavagelloidea
- Pandoroidea
- Verticordioidea
- Poromyoidea
- Cuspidarioidea
|
- Subclass Anomalodesmata
- Family Cuspidariidae
- Family Lyonsiidae
- Family Pandoridae
- Family Periplomatidae
- Family Thraciidae
|
|
In
summary, the taxonomy of the Pelecypods (bivalves, lamellibranches) is a twisted,
complex affair, to be tackled at your own risk!
|
Classification:
|
| The Bivalves consists
of five Subclasses, accounting for some 15,000 known species. I will be
using the OBIS (see above) taxonaomic classification
system to further discuss these subclasses. |
NOTE:
Where possible in the following section, I will be showing a single representative
species in each of the families listed. Occasionally, in cases of a shell being
very rare and I am unable to provide an image but there is a web site to refer
to , I will them list and link to that site. As with all links, that are not
permanent in today's world of change. Should you find a broken link; I sure
would appreciate it if you could notify me of this.
Thank you: Avril Bourquin
|
1.
Subclass Protobranchia: (Pro-to-branch-ia)
Latin: proto=front branch=gill:
Primitive bivalves,
their gills are not folded. Palpal proboscides are frequently present.
|
| |
| |
Order
Nuculoida:
Shell
is aragonic with an interior that is nacerous or porcelaneous The periostracum
is smooth. The valves are equal and have a row of sharp teeth along its
hinge or border. Large palps used for food collection. Ctenidia are small
and used only for gas exchange. Foot is longitudinally grooved and has
a plantar sole. (Common Name: Nut Clams)
|
| |
Superfamilies,
Families & Genus:
- Nuculanoidea
- Nuculanidae
- Ledellinae,
Unplaced, Nuculaninae, Yoldiinae
- Malletiidae
- Malletia,
Pseudomalletia, Bathymalletia
- Sareptidae
- Neilonellidae
Nuculanoidea

Nuculana elenensis
(Sowerby, 1833)
12 mm
|
Malletiidae

Malletia cumingii (Hanley, 1860)
15 mm
|
Sareptidae
Unable to locate a shell or photo
|
Neilonellidae

Neilonella dubia Prashad, 1932
6 mm
|
- Nuculoidea
- Nuculidae
- Nuculinae
- Nucula,
Acila, Pronucula, Nuculoma, Ennucula, Rumptunucula
Nuculoidea

Nucula
semiornata
(Orbigny, 1846)
5 mm up
|
Tindariidae

Tindaria
striata
(King & Broderip, 1831)
12 mm up
|
|
|
Order
Solemyoida:
Shell valves are thin, equal in size, elongate and lacking hinge teeth.
They have a large ctenidae used for both feeding and gas exchange. Their
palps are small.
(Common Name: awning clams)
|
| |
Superfamilies,
Families & Genus:
Solemyidae

Solemya togata
(Poli, 1795)
44 mm
|
Nucinellidae
Bulletin
#35
Huxleyia cavernicola sp. nov.
0.9 mm
|
|
|
2.
Subclass: Pteriomorphia: They
possess attenuated, flexed gill filaments. The filaments are incompletely
fused; intercellular junctions are present but the adjacent filaments
are joined only by ciliary tufts. They are primitive bivalves.
(WAS: Superorder:
Filibranchia:
(Phil-I-branch-ia): (also known as Pteriomorphia):
Latin: fil=thread branc=gill
|
| |
Order
Mytiloida:
Shell valves are quite thin, elongated and equal in size.. The valves
are uncalcified along the outer edges and hinge teeth are absent. They
have one large ctenidia which is used both for feeding and gas exchange.
Their palps are small.
|
| |
Families &
Genus:
- Mytilidae: these
are the sea mussels
- Mytilus,
Brachidontes, Mytilaster, Perna, Crenella, Gregariella, Modiolarca,
Musculus, Rhomboidella, Lithophaga, Myoforceps, Modiolus, Amygdalum,
Dacrydium, Idas, Modiolula, Musculista, Xenostrobus, Bathymodiolus

Mytilus californianus
(Conrad, 1837)
100 mm. |

Perna viridus
(Linnaeus,
1758)
50 mm. |
|
|
Order Arcoida:
|
| |
Superfamilies,
Families & Genus:
- Arcoidea
- Noetiidae
- Noetinae
- Sheldonella,
Noetiella, Estellacar, Verilarca, Spinearca, Didimacar,
Striarcinae
- Striarcinae
- Arcidae
(mainly found in sand or mud in shallow and deep water, attached
to a rocky or coralline substrate by means of a byssus.)
- inquirenda,
Arca, Trisidos, Scaphula, Barbatia, Anadara, Bathyarca, Bentharca,
Porterius, Samacar, Hawaiarca, Mosambicarca
- Cucullaeidae
Noetinae
Noetia
reversa
(Sowerby, 1832)
33 mm.
|
Arcidae

Arca navicularis
(Bruguière, 1789)
27 mm.
|
Cucullaeidae

Cucullaea labiata
(Lightfoot, 1786)
75 mm.
|
- Limopsoidea
- Philobryidae
- Philobrya, Hochstetteria,
Neocardia, Adacnarca, Cratis, Notomytilus, Cosa, Micromytilus
- Limopsidae
- Limopsis, Lissarca,
Empleconia, Nipponolimopsis,Crenulilimopsis
- Glycymerididae
- Incertae sedis,
Glycymeris, Melaxinaea, Tucetona
|
|
Order Pterioida
|
| |
Superfamilies,
Families & Genus:
- Pterioidea
- Pulvinitidae
- Malleidae
- Vulsella,
Malleus, Neoaviculovulsa
- Pteriidae
- Pteria,
Pinctada, Electroma
- Isognomonidae
- Pinnoide
- Pinnidae
- Pinna,
Atrina, Streptopinna
Pinnidae
 Pinna nobilis
Pinna nobilis
(Linnaeus, 1758)
60 cm.
|
|
|
Order Limoida
|
| |
Superfamilies &
Family:
- Limoidea
- Limidae
- Lima, Limaria,
Limea, Limatula, Ctenoides, Acesta, Limatulella, Divarilima,
Fukama
Limoidae

Lima lima
(Linnaeus, 1758)
40 mm.
|
|
|
Order Ostreoida
|
| |
Superfamilies,
Families & Genus:
- Pectinina
- Pectinoidea
- Spondylidae
-
Pectinidae
-
Pecten, Pedum, Amusium, Chlamys, Decatopecten, Argopecten,
Flexopecten , Lissopecten, Hyalopecten, Nodipecten, Patinopecten,
Semipallium, Mimachlamys, Equichlamys, Mesopeplum, Veprichlamys,
Notochlamys, Delectopecten, Cryptopecten, Anguipecten, Haumea,
Mirapecten, Volachlamys, Juxtamusium, Annachlamys, Gloripallium,
Excellichlamys, Bractechlamys, Minnivola, Coralichlamys,
Serratovola, Somalipecten, Pseudohinnites, Glorichlamys
-
Entoliidae
-
Propeamussiidae
- Propeamussium,
Parvamussium, Cyclopecten, Cyclochlamys, Similipecten
Spondylidae

Spondylus americanus
(Hermann, 1781)
Photograph by Jim Miller
10 cm.
|
Pectinidae
Chlamys senatoria nobilis
(Reeve, 1852)
7.5 cm.
|
Entoliidae
Unable
to locate a shell or photo
|
Propeamussiidae

Cyclopecten zacae
(Hertlein, 1935)
(Rare-deep water)
14 mm.
|
- Anomiidae
- Anomiinae
- Placunanomiinae
- Placunidae
Anomiidae
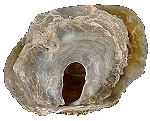
Anomia ephippium
(Linnaeus, 1758)
65 mm.
|
Placuniidae

Placuna sella
(Gmelin, 1791)
19 cm.
|
- Ostreina
- Ostreoidea
- Ostreidae
- Ostreinae
- Ostrea,
Pretostrea, Planostrea, Booneostrea, Pustulostrea,
Nanostrea
- Lophinae
- Lopha
, Dendostrea, Alectryonella, Anomiostrea
- Crassostreinae
- Crassostrea,
Saccostrea, Striostrea
- Gryphaeidae
- Pycnodonte,
Hyotissa, Neopycnodonte, Parahyotissa
Ostreidae

Lopha
cristagalli
(Linnaeus, 1758)
9 cm.
|
Gryphaeidae
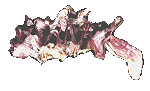
Hyotissa imbricata
(Lamarck, 1819)
7.5 cm.
|
Plicatuloidae

Plicatula gibbosa
( Lamarck, 1801)
11 mm.
(Jaxshells)
|
- Dimyoidea (freshwater
mussels)
Dimyoidea
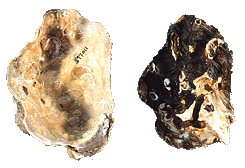
Etheria elliptica
Lamarck, 1807
|
|
|
3.
Subclass: Palaeoheterodonta
(WAS:
Order: Paleoheterodonta: There are about 1,200 species
and it includes the nearly extinct family Trigoniidae (fewer than 6 living
species) and the Unionoidea (fresh water bivalves)
|
| |
Order Trigonioida
|
| |
Superfamily, &
Genus:
Trigonioidea
 Neotrigonia
bednalli
Neotrigonia
bednalli
(Verco, 1907)
5 cm.
|
|
|
Order Unionoida
|
| |
Superfamilies,
Families:
- Unionoidea
- Hyriidae
- Incertae sedis,
Hyridella, Alathyria, Cucumerunio, Lortiella, Velesunio, Westralunio
- Hyriidae2
- Hyridella2,
Lortiella2, Velesunio2, Alathyria2, Westralunio2,
Cucumerunio2
- Unionidae
Muteloidae

Iridina spekei
(Woorward, 1880)
99 mm.
|
|
|
4. Order Heterodonta
('het-er-o-'dän -ta)
(Was
Eulamellibranchia (Eu-la-melli-branch-ia)
Latin: eu=well, very lamella=leaf, layer
branch=gill (also called the Paleoheterodonta and the Heterodonta - two
subclasses often just lumped together as the Eulamellibranchs (and no,
I don't know why nearly every taxonomic group in the bivalves has at least
two names!!) The reflexed gill filaments are morphologically
fused to form true lamellae. )
|
| |
Order Veneroida: Usually
thick-valved, equal valved and isomyarian.
|
| |
Superfamilies,
Families & Genus:
- Chamoidea
- Chamidae
- Incertae, Chama,
Pseudochama, Eopseuma, Carditochama
Chamidae
 Chama
lazarus
Chama
lazarus
(Linnaeus, 1758)
56 mm.
|
- Lucinoidea
- Lucinidae (A
large, well-known family of usually white, hard-shelled clams in
which the cardinal teeth are small, and the anterior muscle scar
is narrow and long. There are no long siphons, so the clams make
a tube to the surface with their foot. Many genera and species,
worldwide, shallow to deep water. [from A&D,
p.320])
- Codakia, Loripes,
Lucina, Anodontia, Myrtea, Ctena, Divaricella, Megaxinus,
Austriella, Loripinus, Cavilucina, Phlyctiderma, Callucina,
Bourdotia, Cardiolucina, Lucinoma, Pillucina, Notomyrtea,
Epicodakia, Nevenulora, Wallucina, Monitilora, Divalucina,
Saltocuna, Barbierella, Divalinga, Mesolinga, Rasta, Lamellolucina
- Ungulinidae
- Diplodonta,
Cycladicama, Ungulina, Microstagon, Fellaniella, Numella
- Thyasiridae
- Thyasira, Leptaxinus,
Tauraxinus, Parathyasira, Mayorithyas,
Axinopsida
- Mactromyidae
- Fimbriidae
Lucinidae
 Loripes
lucinalis
Loripes
lucinalis
(Lamarck, 1818)
15 mm.
|
Ungulinidae

Felaniella vilardeboana (Orbigny, 1846)
17 mm.
|
Thyasiridae
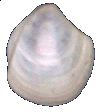
Thyasira trisinuata Orbigny, 1842
9.5 mm
|
Mactromyidae
Unable
to locate a shell or photo
|
Fimbriidae

Fimbria fimbriata (Linnaeus, 1758)
6.2 mm
|
- Galeommatoidea
- Galeommatidae: (a
group with often internalized shells and active crawling behavior)
- Leiochasmea,
Entovalva, Kellia, Lepton, Montacuta, Galeomma, Lasaea, Tellimya,
Myllita, Scintilla, Mysella, Pseudopythina, Lionelita, Scintillula,
Ephippodonta, Issina, Amphilepida, Pseudokellia, Levanderia
, Borniola, Melliteryx, Scintillona, Arthritica, Barrimysia,
Fastimysia, Virmysella, Divariscintilla, Ambuscintilla, Marikellia,
Achasmea, Hitia , Radobornia, Kaneoha, Cicatellia, Parvikellia,Vermitexta,
Fronsella, Pileatona, Paraborniola, Curvemysella, Montacutona
Galeommatidae

Myllita deshayesi
(Orbigny & Récluz, 1850)
9.0 mm. |
- Cyamioidea
- Cyamiidae
- Gaimardia,
Cyamiomactra, Legrandina, Eugaimardia
- Neoleptonidae
- Sportellidae
- Sportella,
Anisodonta, Basterotia, Isoconcha, Tahunanuia
Cyamiidae
 Gaimardia
trapezina
Gaimardia
trapezina
(Lamarck, 1819)
16 mm.
|
Neoleptonidae
 Neolepton
Neolepton
species unknown
2.9 mm.
(Jaxshells)
|
Sportellidae
Unable
to locate a shell or photo
|
- Carditoidea
- Carditidae
- Incertae
sedis
- Cardites
tankervillii, Chama australis, Cardita jukesi,
Cardita (Venericardia) pelseneeri
- Cardita, Beguina,
Venericardia, Cardites, Glans,
Arcinella, Cardiocardita, Thecalia, Cyclocardia, Carditella,
Megacardita, Choniocardia, Hamacuna, Carditellopsis
- Condylocardiidae
- Condylocardiinae
- Micromeris,
Condylocardia, Benthocardiella, Condylocuna, Cunanax,
Austrocardiella, Isodontocardia
- Cuninae
- Cuna, Propecuna,
Ovacuna, Warrana, Crassacuna,
Mimicuna, Westaustrocuna
Carditidae

Cardites bicolor
(Lamarck, 1819)
40 mm.
|
Condylocardiidae

Cuna gambiensis
( Nickles, 1955)
25 mm. |
- Crassatelloidea
- Crassatellidae
- Incertae sedis
- Crassatella
striata, Crassatella subradiata, Crassatella erycinea, Crassatella
corbuloides, Crassatella jubar, Crassatella lapidea, Crassatella
ornata, Crassatella triquetra, Crassatella ziczac, Crassatella
compta, Crassatella concinna, Crassatella obscura, Crassatella
truncata, Crassatella crebrilirata, Crassatella subquadrata,
Crassatella indica
- Eucrassatella,
Talabrica, Salaputium, Bathytormus
Crassatellidae

Crassatella brasiliensis
(Dall, 1903)
(A
rare deep water species)
23 mm.
|
- Cardioidea
- Cardiidae
- Incertae sedis
- Cardium laevigatum,
Cardium rigidum, Cardium (Trachycardium) arenicola, Cardium
hiulcum, Cardium fornasinianum, Cardium istmicus, Cardium
septuagenarium, Vepricardium monilectum, Cardium (?Fragum)
centumliratum, Cardium (Ctenocardium) translatum, Clinocardium
californiense bulowi, Cardium (Cerastoderma) iranjanense,
Laevicardium (Vepricardium) rudentis
- Protocardiinae
- Nemocardium,
Lyrocardium, Frigidocardium
- Cardiinae
- Incertae
sedis, Acanthocardia, Bucardium, Parvicardium,
Plagiocardium, Papillicardium, Rudicardia, Vepricardium
- Trachycardiinae
- Trachycardium,
Acrosterigma, Vasticardium
- Fraginae
- Fragum,
Corculum, Lunulicardia, Ctenocardia, Afrocardium,
Microfragum
- Laevicardiinae
- Cerastoderma,
Laevicardium, Fulvia, Keenocardium
- Hemidonacidae
Cardiidae
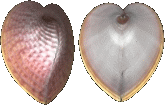
Corculum laevigatum
(Lightfoot, 1786)
36 mm.
|
Hemidonacidae
Unable
to locate
a shell or photo
|
- Tridacnoidea
- Tridacnidae (Giant
clams (Cardiidae: Tridacninae) are among the most familiar marine
invertebrates. Less well known is the remarkable fact that they
are highly derived cardiids (cockles) whose adult morphology has
been profoundly restructured by their long evolutionary association
with photosymbionts. They have been severly over-harvested throughout
much of their collective range and illegal fishing (poaching) remains
a serious problem. )
Tridacnidae

Tridacna (Tridacna) gigas
( Linnaeus, 1758 )
up
to 1.5 meters in length 333kg in weight |
- Mactroidea
- Cardiliidae
- Mactridae: Surf
clams like to burrow in rocks, and the shell is consequently very
strongly constructed.
- Incertae sedis
- Mactra cornea,
Mactra opposita, Mactra sulcataria, Mactra symmetrica, Mactra
incerta, Mactra, Mactra, Mactra taprobanensis, Mactra thaanumi
- Mactrinae
- Mactra,
Spisula, Mactrinula, Diaphoromactra
- Lutrariinae
- Lutraria,
Heterocardia, Meropesta
- Zenatiinae
- Pteropsellinae
- Mesodesmatidae
- Incertae sedis
- Caecella
transversalis, Paphia macrodon, Mesodesma intermedia
- Mesodesmatinae
- Davilinae
Cardiliidae
Unable
to locate
a shell or photo
|
Mactridae

Mactrellona exoleta
(Gray 1837)
|
Mesodesmatidae

Mesodesma
donacium
(Lamarck, 1818)
9 cms.
|
- Solenoidea
- Pharidae
- Siliqua, Cultellus,
Phaxas, Ensiculus
- Solenidae
- Pharellidae
Pharidae

Cultellus attenuatus
(Dunker, 1862)
9 cm.
|
|
Pharellidae

Pharella javanica
Lamarck, 1818)
|
- Tellinoidea
- Tellinidae (Tellin
Clams)
- Tellininae
- Tellina,
Peronaea, Angulus, Phylloda, Tellinides, Tellinella, Tellidora,
Homalina, Eurytellina, Pharaonella, Serratina, Abranda,
Macomona, Obtellina, Clathrotellina, Hemimetis, Arcopella,
Laciolina, Florimetis
- Macominae
- Gastrana,
Macoma, Macalia, Psammotreta, Apolymetis, Leporimetis
- Strigillinae
- Arcopagiinae
- Arcopagia,
Fabulina, Pseudarcopagia, Quadrans, Cyclotellina, Moerella,
Merisca, Arcopaginula, Scutarcopagia, Exotica, Semelangulus,
Pinguitellina, Quidnipagus, Jactellina, Punipagia, Pistris,
Cadella, Loxoglypta
- Psammobiidae
(Sunset Clams)
- Asaphis,
Hiatula, Sanguinolaria, Gari, Psammacola, Soletellina,
Heteroglypta, Psammotellina, Psammosphaerica
- Donacidae
(Donax Clams)
- Donax, Hecuba,
Heterodonax
- Solecurtidae
(Solecurtus Clams)
- Solecurtus,
Azorinus, Sinonovacula
- Semelidae
- Semele, Abra,
Ervilia, Cumingia, Theora, Iacra, Leptomya,
Lonoa, Abrina, Thyellisca, Leptomyaria
- Arcticoidea
- Vesicomyidae
- Vesicomya,
Callogonia, Calyptogena
- Kelliellidae
- Trapeziidae
- Trapezium,
Coralliophaga, Fluviolanatus
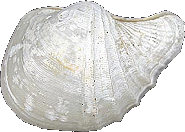
Vesicomyidae
 Calyptogena magnifica
Calyptogena magnifica
(Boss & Turner, 1980)
(This is a Deep Sea Vent Clam)
[Collection of Emilio
Jorge Power]
|
Kelliellidae
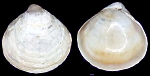
Alveinus ojianus
(Yokoyama, 1927)
(shell is only 0.5mm.)
|
|
- Glossoidea
Glossidae

Glossus humanus
(Linnaeus, 1758)
9 cm.
|
- Corbiculoidea
(freshwater)
- Incertae sedis
- Cyrena
eximia, Batissa triquetra, Cyrena affinis, Cyrena fallax,
Cyrena oviformis, Cyrena ventricosa, Batissa australis, Cyrena
essingtonensis, Cyrena impressa, Cyrena jukesi, Cyrena placida,
Cyrenella moretonensis, Cyrenella sphaericula, Corbicula minor,
Corbicula prolongata, Cyrena cyprinaeformis, Corbicula baronialis,
Cyrena rugulosa, Cyrena solida, Corbicula deshayesii, Corbicula
desolata, Corbicula faba, Corbiculina aramita, Corbiculina
esculenta, Corbiculina finkeana, Corbiculina musson, Corbiculina
semara
- Polymesoda,
Batissa, Cyrenobatissa
Corbiculidae

Corbicula fluminea
(Müller, 1774)
|
- Veneroidea
- Petricolidae
- Petricola,
Mysia, Asaphinoides
- Veneridae (Venus
Clams)"The family Veneridae has over 400 living species.
It is one of the most colorful of the bivalve groups. Shape varies
from circular to triangular, and from side view will appear as either
ovate (egg-shaped) or cardioid (heart shaped) shells. Characterististics
are: porcelain-like shell that is highly finished; a complex tooth
structure in the hinge; a well developed escutchion and lunule;
and, a well developed sinus at the pallial line. Spiny forms are
comparatively uncommon in this family..."
- Incertae
sedis
- Venerupis
flabagella, Pitar (Pitar) varians, Venus maculata, Venus
punctata, Cytherea concentrica var., Cytherea rufa, Venus
truncata, Venus variflamma, Venus tessellata, Circe planata,
Venus bella, Artemis turgida, Tapes geographica, Chione
mitis, Venus fluctifraga, Saxidomus maximus, Venus consobrina,
Venerupis chinensis, Venerupis digonea, Chione gibbosula,
Chione sphaericula, Chione ustulata, Tapes similis, Dione
crocea, Dione rufescens, Meretrix grata, Venus deshayesiana,
Circe robillardi, Dosinia conglobata, Dosinia minor, Circe
undata, Cytherea ambigua, Gouldia petterdi, Sunetta clessini,
Circe gordoni, Callocardia (?) pacifica, Circe amica, Dosinia
parva, Callista (Callocardia), Dorisca cookei
- Unplaced
- Venerinae
-
Venus, Antigona, Periglypta, Globivenus
- Dosiniinae
- Sunettinae
- Meretricinae
- Tapetinae
- Paphia,
Tapes, Irus, Venerupis, Gomphina, Katelysia, Marcia, Ruditapes,
Granicorium, Eumarcia
- Circinae
- Gafrarium,
Circe, Gouldia, Circenita, Microcirce
- Pitarinae
- Callista,
Lioconcha, Pitar, Callocardia, Amiantis
- Clementiinae
- Chioninae
- Anomalocardia,
Timoclea , Clausinella, Chamelea, Protothaca, Bassina,
Placamen, Tawera
- Samaranginae
- Glauconomidae
Petricolidae

Petricola denticulata
(Sowerby, 1834)
20 mm.
|
Veneridae

Callista florida
(Lamarck, 1818)
33 mm.
|
Glauconomidae

Glauconome chinensis (Gray, 1828)
20 mm.
|
|
|
Order Myoida:
Thin-shelled, burrowing bivalves with well developed syphons.
Includes the families:
|
| |
Superfamilies,
Families & Genus:
- Myoidea
- Myidae
- Sphenia, Tugonia,
Cryptomya, Tugonella
- Corbulidae
- Bithinella, Corbula,
Potamocorbula
Myidae

Sphenia hatcheri
(Pilsbry, 1899)
13
mm.
|
Corbulidae

Corbula caribaea
( Orbigny, 1842)
8 mm.
|
- Gastrochaenoidea
- Gastrochaenidae
- Gastrochaena,
Rocellaria, Spengleria, Cucurbitula, Eufistulana
Gastrochaenidae
 Gastrochaena
cuneiformis
Gastrochaena
cuneiformis
(Spengler, 1783)
15 mm.
|
- Hiatelloidea
- Pholadoidea
- Pholadidae
- Pholas, Pholadidea,
Martesia, Barnea, Jouannetia, Penitella
Parapholas, Aspidopholas, Nipponopholas
- Teredinidae:
The Teredinidae (shipworms) live in calcareous tubes which erode
the wooden structures they live in.
- Teredininae
- Teredo,
Uperotus, Kuphus, Bankia, Nausitora, Lyrodus, Bactronophorus,
Teredora, Teredothyra, Nototeredo, Spathoteredo, Dicyathifer
Pholadidae
 Pholadidea
melanura
Pholadidea
melanura
(Sowerby, 1834)
40 mm.
|
Teredinidae

Teredo
navalis
(Linnaeus, 1758)
12 mm.
|
|
(Note on Shipworms: teredos
are specialized molluscs that bore into wood. They are not a worm
at all, but a greatly elongated clam . Its two shells enclose
only the front end of the body and function as a tool, (rather
than a protective covering) and the shell's ridged and roughened
surfaces are used for boring into the wood. The burrow is lined
with a calcareous coating produced by the clam's mantleand is
begun when the animal is in its larval stage and it continues
to expanded as it grows. The common shipworm of the Atlantic Ocean,
Teredo navalis, may grow up to 2 ft (60 cm) long, although its
shells remain only 12 in. (12 mm) long.
|
|
|
Shipworms then feed on wood particles
and minute organisms found in the wood. Unlike most shipworms
Teredo navalis is able to subsist on wood alone. They do enormous
damage to piers and other wooden structures found in the water.
In the days of wooden ships they would eat right through hulls
thus sinking the ship! . It was not until the discovery that placing
a skin of copper plate on the hull of these wooden ships was the
problem controlled. In the 15th and 16th century, the only way
to protect a ship was to cover the hull with tar and pitch. This
would work for a short time, and the ship had to be beached, periodically
so that infested timbers could be replaced and re-pitched.)
|
|
|
5. Subclass:
Anomalodesmata
(A-nom-o-des-mat-a)
A small, specialized group, in which gills are not present.
The inhalent and suprabranchial (exhalent) cavity are separated by a pumping septum.
(WAS:
the Septibranchia (Sept-a-branch-ia)
Latin: sept=seven, wall branch=gill (also
called Anomalodesmata
|
| |
Order Pholadomyoida
|
| |
Superfamilies,
Families & Genus:
- Pholadomyoidea
- Pholadomyoidea
- Pholadomyidae
(Piddock Clams)
- Parilimyidae
Pholadomyidae

Pholadomya candida
(J. Sowerby, 1823)
94 mm.
|
Parilimyidae
Unable
to locate a shell or photo
|
- Thracioidea
- Thraciidae
- Thracia,
Phragmorisma, Thraciopsis, Parvithracia
- Periplomatidae
- Laternulidae
Thraciidae

Thracia
similis
(Couthony, 1839)
20 mm.
|
Periplomatidae

Periploma angasi
(Crosse & Fisher, 1864)
49 mm.
|
Laternulidae

Laternula anatina
(Linnaeus, 1758)
75 mm.
|
- Pandoroidea
- Lyonsiidae
- Pandoridae
- Myochamidae
- Verticordioidea
- Euciroidae
- Verticordiidae
- Verticordia,
Haliris, Halicardia, Thracidora, Spinosipella, Vertambitus,
Policordia
- Lyonsiellidae
Euciroidae

Acreuciroa rostrata
|
Verticordiidae

Vertambitus pygmaea
( Kuroda, 1952)
|
Lyonsiellidae
Unable
to locate
a shell or photo
|
- Poromyoidea
- Poromya, Cetoconcha
Poromyidae

Cetoconcha eximia
(Pelseneer, 1911)
12 mm.
|
- Cuspidarioidea
- Cuspidaria, Cardiomya,
Halonympha, Myonera, Pseudoneaera, Protocuspidaria
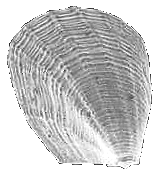
Cuspidariidae
 Cardiomya
alcocki
Cardiomya
alcocki
(Smith, 1884)
11 mm.
VERY RARE ribbed shell, deep water (1000 meters) |
|
(Diagram)
The
typical bivalve shell consists of two similar, convex and oval or elongate
valves. These valves are attached and articulate with
each other. The shell is made up of three layers: The periostracum
or thin outer layer that is made of horny, organic material called conchiolin,
the prismatic or thick middle layer that is made up of calcium carbonate crystals
arranged in vertically, and the nacerous
or thinner inner layer that is composed of thin horizontally arranged calcium
carbonate crystals. (Diagram)
Dorsally,
the shell has a protrusion called the umbo, which rises above
the articulation (most commonly called �teeth �). The umbo
is the oldest part of the shell. The concentric lines found around the umbo
are growth lines, which are usually seasonal, making it a lot easier to tell
the age of a bivalve than a gastropod! The two valves are attached by
an elastic band of cartilage-like material called the hinge ligament, which
is made up of conchiolin -
the same as the periostracum. The hinge ligament is designed to hold both halves
of the shell together. The main muscle of a bivalve is called the adductor muscle,
and is also used to hold the shell halves together. When the bivalve �s
adductor muscles
relax, the ligament causes the valves to open. Most species are also equipped
with locking teeth or sockets beneath the ligament to prevent lateral slippage.
The valves are pulled together through the action of the two strong, adductor
muscles. They are antagonistic to the hinge ligament, as just explained.
On the inside of the shell is a scar that marks where these muscles attach.
In
most bivalves, the valves are similar in structure and size; however, in a few
families such as the oysters and jingle shells the upper or left valve is always
larger than the right valve. For those bivalves that attach to a substratum,
they always do this by their right valve, which is always the smaller of the
valves, if there is a difference between them.
In
the more ancient bivalves, the adductor
muscles are of equal size. Many families have evolved to the point where
the anterior adductor
has reduced in size and in some, like the oysters, it has disappeared all together.
In these cases, the posterior adductor has shifted to a more central location
between the valves. (This large muscle is the part most often eaten by man -
for example, the round, white meat we often call a �scallop � is
in fact on the scallop's adductor muscle.)
Some
bivalves can rapidly shut their two valves. This is to be found in scallops,
for example. Here, the adductor muscle is divided into one section of
striated fibers and one section of smooth fibers. The striated fibers cause
the rapid closing and the smooth fibers sustain the contraction.
The bivalve shell exhibits a great variety of shapes, sizes, surface sculpturing
and colours. In size they range from a few millimeters (<2mm in the Sphaeriidae)
to over four feet (1.3+ meters in the Tridacna gigas). They come in all
colours and colour combinations, and range from smooth as glass to having long
sharp spines in their sculpturing (e.g.: the Spondylidae, or "spiny
oysters".)
The mantle greatly overhangs
the soft body and forms a large sheet of tissue lying beneath the valves. The
edge of the mantle has three folds; an outer, middle and inner one. The
innermost fold is the largest and contains radial and circular muscle tissue.
The middle fold acts as a sensory organ. The outermost layer is responsible
for secreting the shell. The inner surface of this outer fold lays down
the periostracum,
and the outer layer lays down the prismatic and nacerous shell layers. The nacerous
layer is also secreted by the entire outer surface on the mantle.
The
mantle is attached to the shell, in a semicircular line just inside the shell
edge, by means of the inner lobes' circular muscle. This attachment leaves
a visible scar on the inside of the shell known as the "pallial line".
The pattern of the pallial lines and adductor muscle scars is extremely
useful in identifying very similar species, when you only have the shell.
This attachment prevents foreign particles form getting lodged between the mantle
and the shell. However; sometimes a foreign substance such as a grain
of sand or parasite does get in. To prevent this from irritating the mantle,
the mantle lays down concentric layers of nacerous shell around the particle.
This is how a pearl is formed. Sometimes the pearl becomes totally embedded
in the shell itself. Most bivalves are capable of forming a pearl; however,
it is the pearl oyster, Pinctada margaritifera that produces the finest
natural pearls man uses for jewels. Cultured pearls are started when man
intentionally inserts a nucleus (a microscopic globule of liquid or solid irritant)
in an oyster. When this pearl is approximately one year old and has a
covering of 1 millimeter, this seed pearl is transplanted into another oyster.
Three years after this transplant, the pearl is usually marketable.
To
facilitate burrowing into the mud or sand where bivalves live, the foot has
evolved to become compressed and blade-like. In the more primitive Protobranchia
the foot has a flattened sole on its foot. The edges of this sole fold
together to form a sharp edge. It thrusts this sharp-edged foot into the sand
or mud then it opens it up again so that the foot now acts as an anchor and
the remaining body is pulled down into the soft substratum. Some of the
other bivalves, without this flat sole, can inflate the leading edge of the
foot: it then acts as an anchor and is used for digging-in in much the
same fashion.
Bivalve foot movement is accomplished through a combination of changing blood
pressures and muscle action. Attached to the shell, just below the anterior
adductor muscle is a pair of protractor muscles that extend from each side of
the foot and attach to the opposite valve. Blood engorges the foot, increasing
its blood pressure. This increased blood pressure in conjunction
with the "pedal protraction muscles" (the muscles which manipulate
the foot), causes extension. Withdrawal of the foot is effected by the
contraction of a pair of posterior retractors also attached to the foot and
shell, and by the contraction of muscle fibers in the foot itself: in other
words, the bivalve sort of "inchworms" its way around by contracting
and expanding its foot muscle, thereby withdrawing and extending it using a
combination of blood-pressure changes and muscles.
Some bivalves, such as the Cockles (Cardiidae (Cardium)), move
along the bottom by means of jumping. Here the foot is extended then contracted
violently, moving backwards in the process. Thus, the foot acts like a
spring always kicking the animal slightly forward.
The sessile bivalves, such as the oyster and jingle shells (Anomia),
have a greatly reduced foot. Scallops also have a reduced foot and swim
in jerky, but often quite effective in the short run, movement through the water
by slamming shut their valves. This sudden closure causes two streams
of water to be expelled rapidly from each side of the hinge, causing a form
of "jet propulsion".
The mussels (Mytilidae) live attached to rocks, shells, man-made
structures such as piers, or other mussels. They stay attached by means of strong
horny threads called byssal threads (Diagram). A gland in
the foot of the mussel produces these threads. This gland, which is situated
just above and behind the small round foot, produces a secretion that flows
down the back side of the foot and out to the tip of the foot, which is in contact
with the hard substratum. This secretion runs onto the substratum where it hardens
as it comes in contact with the water. A thread is formed. The foot then
withdraws and this process is repeated many times on a slightly different area
of substratum. A web of byssal threads thus holds the mussel fast.
| Water
Circulation & the Mantle Cavity |
The
bivalve body has become greatly lengthened dorsal-ventrally (i.e., it has been
flattened), and this flattening, in combination with an overhang of the shell,
creates an extensive mantle cavity. The mantle cavity extends anteriorly (i.e.,
to the front), and to each side of the body.
In some primitive Protobranchs, inhalent
water enters the mantle cavity anteriorly, passes over the gills and exits posteriorly.
Since these bivalves live buried, sediments get pulled in with the inhalent
water. Cilia lining the mantle cavity, foot and gills sweep these sediments
to the mantle edge where it accumulates. Every now and then the valves
contract rapidly and flush these sediments out. Some, such as the Nucula,
also have �hypobrachial � glands for consolidating the finer sediments
that pass through their gills.
Drawing sediment in with the inhalent water was a major problem for the burrowing
bivalves and the solutions made by the primitive species in the Protobranchia
group was not very efficient. More advanced bivalves adopted several fundamental
strategies to overcome this problem: In all the bivalves as well as in
most of the Protobranchs, the inhalent current returned to the posterior end.
Water enters posteriorly and ventrally, then makes a U-turn through the gills,
and passes back out posteriorly and dorsally. This enables the bivalve
to burrow their anterior end into the soft substratum, leaving just the elongated
inhalent posterior end protruding through the sediment, clear of excessive sand,
mud or silt.
The second change came about with the sealing off of the
mantle edges where openings are not necessary. This in turn led to
the development of the inhalent and exhalent siphons.
The mantle edges surrounding these fused edges are often elongated to form actual
tubular siphons of varying lengths. This system is very advantageous, as the
animal can now remain buried in the sediments with only the tip of its siphon
protruding. The siphon can also be retracted by means of the siphon retractor
muscle that was derived from muscle tissue of the innermost mantle fold. (The
pallial sinus markings on the shell show where this siphon was to be found.)
There is a lot of variation to be found amongst different bivalves as to the
size, length and shape of this siphon. Some are short and poorly developed
while others are very long and so big that they can no longer be contracted
into the shell. Some species have inhalent and exhalent siphons the same length
while in others they are quite different. In length
In
some, the mantle fusion has been carried to a point where only three apertures
are now present. One aperture for each of the inhalent and exhalent siphonal
canals and one for the foot. A few have a fourth aperture through which
the byssal threads
pass.
Most
bivalves have one pair of long gills that separate the mantle cavity into a
ventral inhalent chamber and a dorsal exhalent chamber (also known as the �suprabranchial �
chamber, for those that like long, fancy words!!).
Cilia
provide the power to bring water into the inhalent chamber. Sediment that
enters with the inhalent water gets trapped on the lateral cilia and
is swept by the frontal cilia to the midline. It is then moved anteriorly (towards
the front) along the gill to the foot (which is, ironically, in the front of
the bivalve!!). The sediments are then deposited along the ventral (i.e., bottom)
mantle edge. Periodically the valves snap shut, flushing these sediments
out. Fine sediment particles, however, can pass through the gill
and they become trapped in the secretions of the hypobrachial glands located
over the exhalent chamber.
In
the primitive order of Protobranchs (Diagram), the gill is
not folded and palpal proboscides are frequently present. This is the
case in the Yolida, Solemya, Nucula, Nuculana, and
Malletia.
Other ancient bivalves appear to have a double set of gills. The second
sets of gills actually arise from a folding of the single gill. This is
the case in the Filibranchia and Eulamellibranchia, where the gills have also
taken over the function of obtaining food. To do this, the gills have
many more and greatly lengthened cilia on the gill surface. These long cilia
projected somewhat arterially and then become slightly flexed (bent) downward
in the middle. At the angle of the bend, an indentation or notch is formed.
The notches on the adjacent cilia all line up and form a "food groove"
that extends along the underside of the gill. As they developed through
time, this flexion increased until the cilia became U-shaped. Cilia on
both sides of the axis, now folded in two, became known as the ascending limb
and descending limb. This transformed the single gill into a pair of gills in
these bivalves and they became known as the demibranchs.
In the Filibranchia, bars of tissue, called interlamellar
junctions, grew between the two limbs of each U at intervals. However, the adjacent
cilia still remained attached only by tufts of cilia. Each gill was now
composed of two lamellae and formed a tight mesh.
In these bivalves, the frontal cilia carry the food particles, which are trapped
on the gill surface, downward to the food groove, and the lateral cilia move
the water through the gills. Between the frontal cilia and the lateral
cilia, along the angles of the gill limbs, a new ciliary tract was formed of
lateral and frontal cilia. These cilia prevent large sediments from clogging
the gills.
Inhalent water entering the posterior (back) end of the animal enters the inhalent
chamber. It now flows between the filaments and moves up between the two lamellae.
From the interlamellar spaces, the water flows into the exhalent chamber and
then flows out through the exhalent opening. In this system the hypobrachial
glands became unnecessary as only the very finest of sediments ever pass
through the tightly meshed gills. They eventually disappeared all together.
The order filibranchia with this gill structure includes
the mussels (Mytilus and Modiolus are the mussel genera most
commonly eaten), and Ark shells (Arcidae) the oysters- Ostrea, Crassostrea
and Spondylus; Anomia; Lima; and the scallops (Pectinidae), and
the boring Lithphagia.
In the Eulamellibranchia (Diagram),
the union of filaments even developed further and the ciliary junctions were
replaced by actual fusion. The lamellae now consisted of solid sheets of tissue.
The number of interlamellar junctions also increased. They now extend
the length of the lamellae dorso-ventrally (i.e., from top to bottom, vertically)
dividing the interlamellar space into vertical water tubes. The tips of the
ascending limbs have become fused with the upper surface of the mantle on the
outside and the foot on the inside. This now morphologically separates
the inhalent chamber from the exhalent chamber. Instead of blood oxygen diffusion
occurring through the lamella, the blood is now carried through the lamellae
in vertical vessels that course within the interlamellar junctions. (And
if you can visualize that tangle of concepts, you are pretty smart!!).
Water in the inhalent chamber now circulates between the ridges, and then enters
the water tubes through numerous pores (ostia) in the lamella. Oxygenation
takes place as the water flows dorsally through the tubes. The water then
flows into the exhalent or suprabranchial cavity and out the exhalent opening.
This system was improved upon by many of the Eulamellibranchs. In these
bivalves the surface of the lamella has been increased by folding.
Their gills now have an undulated appearance.
The order of Eulamellibranchia with their gill filaments morphologically fused
includes the Cardiidae (Cockle shells!); the edible Mercenaria
(Quahogs); the boring clams- the Petricola (false Angel Wings), Hiatella,
Martesia and Teredo (ship worms); the razor clams- Tagelus
and Ensis; the little Donax clams; Abra; Pholas
(True Angel wings!); Lyonsia; Macoma; the most common freshwater
clams- Unionidae; Lampsilidae, Anodonta, and Simpsoniconcha;
and the freshwater Sphaeriidae and Magaritidreidae.
In
all bivalves, the inner mantle surface plays some role in oxygenation.
In the Septibranchia (Diagram)
(which includes the Poromyidae, and the little Spoon Clams - Cuspidariidae,
however, the gills have degenerated and modified to become a pair of perforated
(full of holes) macular septa that separate the inhalent chamber and the exhalent
chamber. Muscular contractions of this septum move it up and down,
which causes water to flow into the inhalent chamber and forcing it out the
exhalent chamber. The mantle has in
this order taken over the function of respiration completely.
In
most bivalves, the heart folds around the rectal portion of the digestive system so
that the pericardial sac engulfs the heart as well as a short portion of
the digestive tract. The thin-walled auricles are attached to the muscular
ventricle that surrounds the rectum. Ventricular contractions are strong
and usually quite slow (approximately about 20 per minute). Bivalves exhibit
a typical molluscan circulatory route through the heart, tissue sinuses, nephridia,
and gills. Minor variances do exist among the different orders and
families, but I will not go into these.
The blood is similar to that of the gastropods; however, some such as the Arcidae
(Ark Clams) and Limidae, hemoglobin rather than hemocyanin is present
- so these have red, as opposed to the clear or greenish blood most molluscs
possess.
| Nervous
System and Sense Organs:' (Diagram) |
Bivalves
possess a bilateral and relatively simple nervous system. They have three
pairs of ganglia and two pairs of long nerve cords.
A
cerebropleural ganglia is located on both sides of the esophagus and they are
connected by a short commisure across the top of the esophagus.
From these ganglia two nerve cords travel to a pair of closely adjacent visceral
ganglia located beneath the posterior adductor muscle. Now the second pair of
nerve cords pick up and carry the nerve signal to a pair of pedal ganglia located
in the foot.
Most
bivalve sense organs are located in the margin of the mantle.
Many species possess pallial tentacles, which contain tactile and chemoreception
cells. The entire margin may bear tentacles with, or without eyes (e.g.
Pectinidae and Limidae) but usually these are restricted to the
inhalent or exhalent aperture or siphons or often they fringe the pedal aperture.
A
statocyst is generally found near or embedded in the pedal ganglia. This
statocyst is a small organ of balance and generally consists of a fluid-filled
sac containing statoliths (little stones) that help to indicate relative position.
In
some bivalves, ocelli (small simple eyes) are present along the edge of the
mantle or on the siphons. In the Spondylus and Pectinidae,
the eyes are quite well developed consisting of a cornea, lens and retina.
These eyes most likely cannot form a well - focused image but they can detect
changes in light intensity with the photoreceptor cells found in the ocelli.
Bivalves
also possess an osphradium,
or chemoreception organ which lies directly bellow the posterior adductor muscle
in the exhalent chamber. How this sense organ works is not fully understood
as yet (another thesis topic for you!!)
| Nutrition
& Digestive System (Diagram) |
Most
bivalves are ciliary feeders (or filter-feeders). Their gills have taken
over the role of trapping food particles as well as respiration.
However;
in the ancient order of Protobranchs, the role of food collection is carried
out by the elongation of their mouth structure, which is formed into a
muscular proboscis and a pair of palps
that extend back towards the gills. This proboscis extends into the
surrounding mud or sand and organic detritus is drawn in and carried along its
length by means of ciliary action. It is then passed to the palps where
it passes through the two lamellae. Here the detritus
is sorted and particles for digestion are sent on to the mouth along a
deep oral groove. Rejected particles are swept to the edge of the lamellae
then transferred to the mantle cavity along with the water current.
Food
entering the mouth is passed anteriorly to the stomach via ciliary action.
The stomach is surrounded by a large digestive gland and is divided into two
regions. In the first region (dorsal) the esophagus
and ducts of the digestive gland enter and it contains a ventral style sac.
This dorsal portion of the stomach is lined with chitin except for the large
folded and ciliated sorting region, into which the digestive gland opens.
At the apex of the stomach
is a tooth-like projection called the gastric shield, which arises from the
chitinous girdle. At
the end of this region is the cecum.
Food
is passed along the sorting region of this dorsal section. A few food
particles do enter the digestive gland; however most are passed onto the cecum.
When the food particles pass out of the cecum, they get enmeshed in great masses
of mucus that fills the ventral style sac. This mass is rotated by
the cilia lining the style sac, and along with the muscular action of the
sac, this mass is moved dorsally into the upper region of the digestive tract.
The leading edge of this mucus mass is wound around the tooth-like gastric
shield and is pressed hard against the chitinous girdle. This winding process
causes pieces to be broken off and ground up. Smaller bits are passed
to the digestive gland and coarser bits are passed venrally into a deep
groove along the anterior wall of the style sac and they are then passed directly
into the intestine. (Diagram)
The ducts of the digestive gland are ciliated and are divided into an incurrent
and excurrent tract. Food particles enter the tubules of the digestive
gland via the incurrent tract. Here the particles are engulfed by the
cells of the gland and are digested intracellulary. Wastes are dumped
into the excurrent tract and are moved by ciliary action back to the stomach
where they then get swept into the style sac groove and intestine. The
long intestine loops once or twice around the stomach and then passes through
the anterior adductor muscle and becomes the rectum. The rectum extends
through the heart and pericardial cavity and then opens through the anus
at the posterior of the suprabrachial cavity. The intestine only serves
in the role of forming feces - no absorption takes place here. Feces leaves
as well formed pellets with the exhalent water current.
In the Filibranchia and Eulamellibranchia,
the gills have assumed the function of food acquisition. The proboscides
have disappeared but the lamellae have been retained. These bivalves have
adapted to eating small phytoplankton and very little coarse material ever
reaches the stomach.
Plankton gets trapped in mucous that is on the gill surface and cilia sweep
this mass into the food groove, (some bivalves have both a dorsal
and a ventral
food groove) which runs along the gill. The food is then sorted in the
lamellae and, acceptable food is passed into the mouth and rejected materials
are swept to the ventral edge of the mantle and then posteriorly where
they accumulate behind the inhalent aperture. When the valves periodically
close, these wastes and water are forced out the inhalent siphon.
The acceptable food particles are fine enough that they don't require as much
grinding and the girdle of chitin has become much reduced. The style sac
and the mucous in this group has consolidated to form a very compact, and often
a very long rod called the crystalline style (usually about one inch in length
those of the Tridacna or �giant � clam may reach a length of 13 inches).
This crystalline style in addition to producing its protein matrix also produces
amylase for digestion but basically it acts very much like that to be found
in the gastropods. The projecting tip of the style is rotated by ciliary
action and as grinds against the gastric shield the enzymes are shed into the
food particles. (The style is constantly replaced at its base and it may spin
as rapidly as 11 to 70 times per minute; this rate is affected by temperature,
PH, and food pressure as well as the ciliary action.) This mix passes
through the sorting area of the stomach and the finer particles are moved into
the digestive glands, of which there are from two to twenty. Here digestion
and absorption takes place intracellulary. Any rejected or waste particles
from the digestive gland are passed directly into the intestine.
The
Septibranchia, which have lost their gill structure, have become either carnivorous
or scavengers. The pumping action of their septum provides sufficient negative
pressure to pull in small animals. These animals are seized by the much
reduced but very muscular lamellae and are passed into the mouth. The
stomach is lined with chitin and it acts as a gizzard, crushing
up the animal. The style is also much reduced in this group and may only function
in coating harder particles with mucous to protect the intestine from injury.
In
the bivalves, one oddity does exist, the "giant clam"Tridacna
gigas. This species, besides its regular food procuring and digestive
process, literally farms unicellular algae of the family Zooxanthellae,
which it encourages to grow within its mantle tissue. Some of these algae
get engulfed and are subsequently digested by phagocytic cells thus providing
an additional food source for the Tridacna.
Bivalves
posses two nephridia, which are located beneath or just slightly posterior to
the pericardial cavity. The nephridia are folded to form a long U.
One arm is glandular and opens into the pericardial cavity. The other arm forms
a bladder and opens through the nephridiopore at the anterior of the suprabrachial
cavity.
The
majority of bivalves are dioecious
(two sexes). Their two gonads
are very closely situated next to each other and they encompass the intestinal
loops. The gonoducts are very simple as there is no copulation amongst
bivalves.
In
the Protobranchs and Filibranchs, the gonoducts opens directly into the nephridia
and provide for the exit of sperm and eggs.
In
the Eumellibranchs, the gonoducts opens directly into the mantle cavity very
close to the nephridiopore.
A few bivalves such as
the Cockles (Cardiidae), Poromyidae, a few of the oysters and
scallops (Pectinidae), some of the fresh water clams Sphaeriidae
and Unionidae are hermaphroditic
(one sex).
In most of the bivalves,
sperm and eggs are released into the surrounding water where fertilization occurs.
The eggs and sperm, which were deposited into the suprabrachial chamber, are
swept out along with the exhalent current.
In a few of the bivalves,
such as the common oyster Ostrea edulis L., fertilization occurs within
the suprabranchial chamber itself when sperm is drawn in along with the inhalent
current. The fertilized eggs then develop in the gill filaments.
In
some of the freshwater hermaphrodites, self - fertilization may actually occur
in the genital ducts before the eggs are deposited into the suprabranchial chamber.
The eggs then travel into the water tubes of the gill and there they develop
into larvae.





























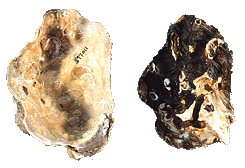

























 Calyptogena magnifica
Calyptogena magnifica